Cathode materials discovery example
Identifying factors limiting capacity
In the example below, the first-cycle capacity loss of Ni-rich NMC (LiNi0.87Mn0.05Co0.08O2) was investigated using charge photometry.
In the example below, the first-cycle capacity loss of Ni-rich NMC (LiNi0.87Mn0.05Co0.08O2) was investigated using charge photometry.
A Li-rich surface develops around a comparatively Li-poor core at the end of lithiation. This non-uniform charge distribution is attributed to the slow diffusion of Li-ions in the nearly fully-lithiated surface, limiting the transport of ions towards the centre of the particle. The cell reaches the cut-off voltage with the particle core still in a Li-deficient state and this behaviour is responsible for the observed first cycle capacity loss.
Taken from Lun, Z. et al. (2025), Energy Environ. Sci. Click on figure to go to original publication.
In a more recent study, charge photometry reveals differences in Li-ion transport dynamics in fresh and aged Ni-rich NMC cathode particles. For aged particles, Li-ion transport is heterogeneous across the particle surface.
The resulting asymmetric delithiation behaviour is attributed to the uneven growth of rocksalt layers and this contributes to capacity and rate degradation, particularly at higher cycling rates. This highlights the importance of promoting uniform delithiation to improve the long-term stability and rate capabilities of Ni-rich NMC cathodes.
This example illustrates how charge photometry is used to understand what is limiting battery material performance. Our charge photometry technology:
NFM data in the figures above and below were captured in collaboration with Professor Chao Xu, Shanghai Tech University
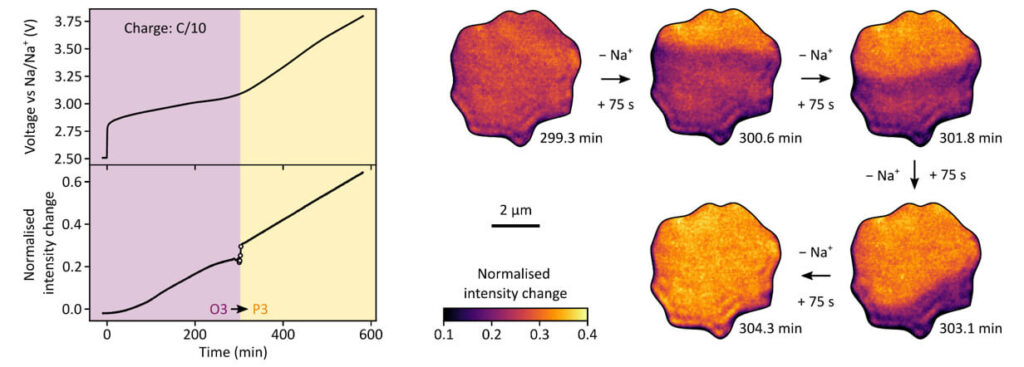
Beyond Li-ion batteries, Na-ion batteries represent a promising alternative chemistry. Charge photometry is material agnostic and hence is ideal for improving the performance of Na-ion battery cathode materials.
In this example, the illumionONE was used to observe Na-ion dynamics in single crystal NFM (NaNi0.33Fe0.33Mn0.33O2) cathode materials. During charging, NFM is progressively de-sodiated, changing its light scattering properties. The images are of a single NFM particle and they reveal a dynamic phase transition during charging, visualised as a bright orange wavefront progressing through the particle from top to bottom.
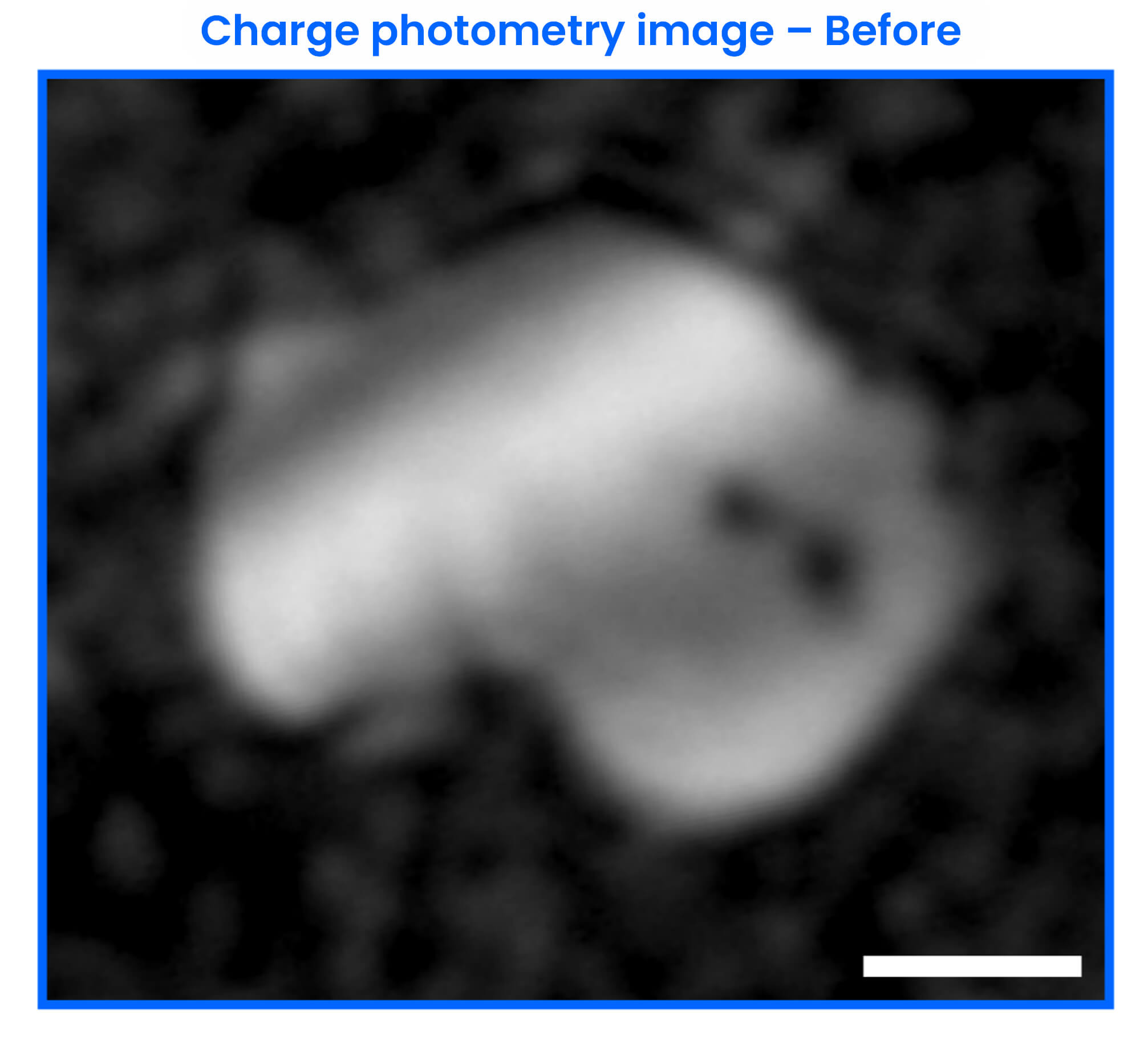
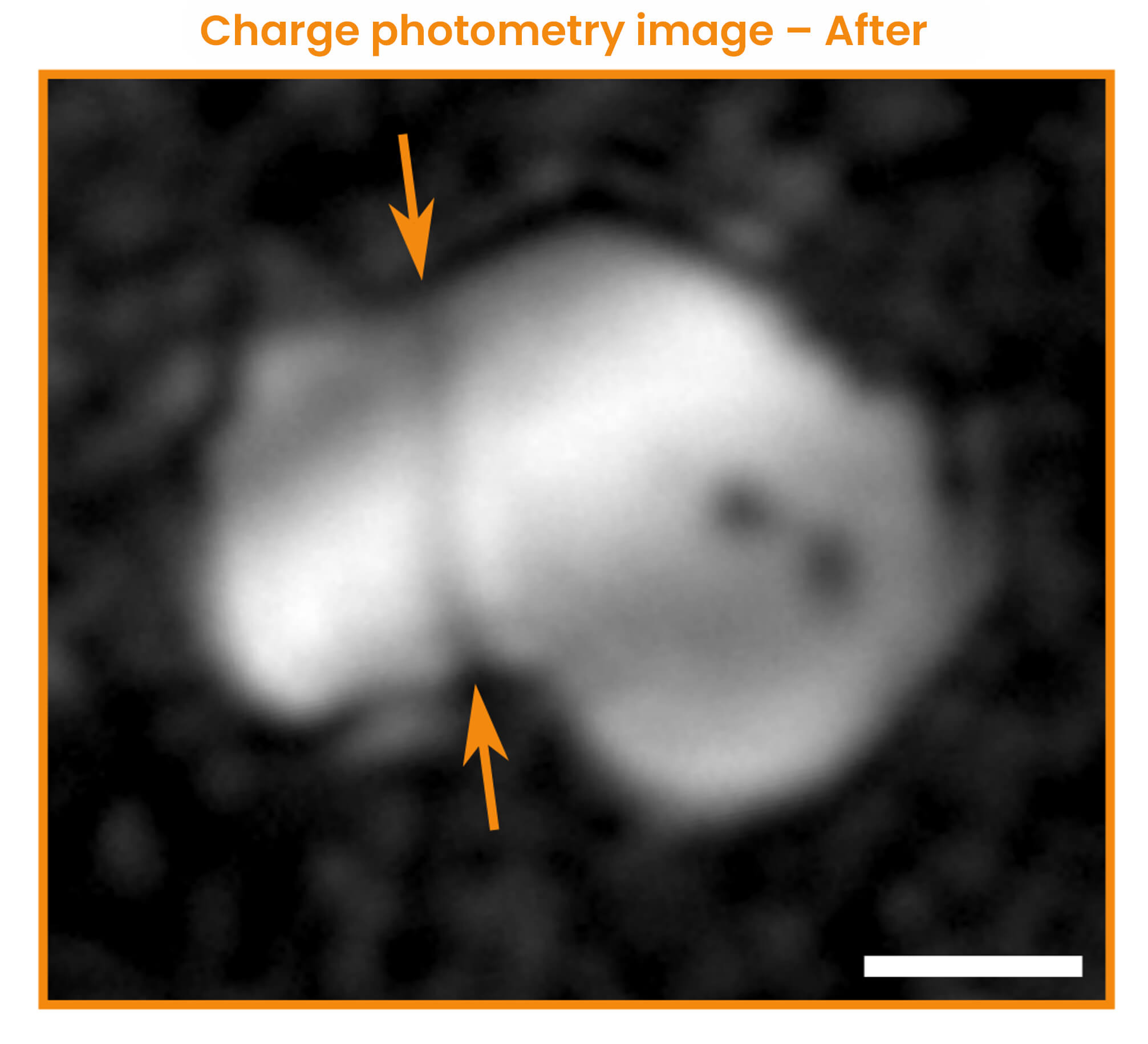
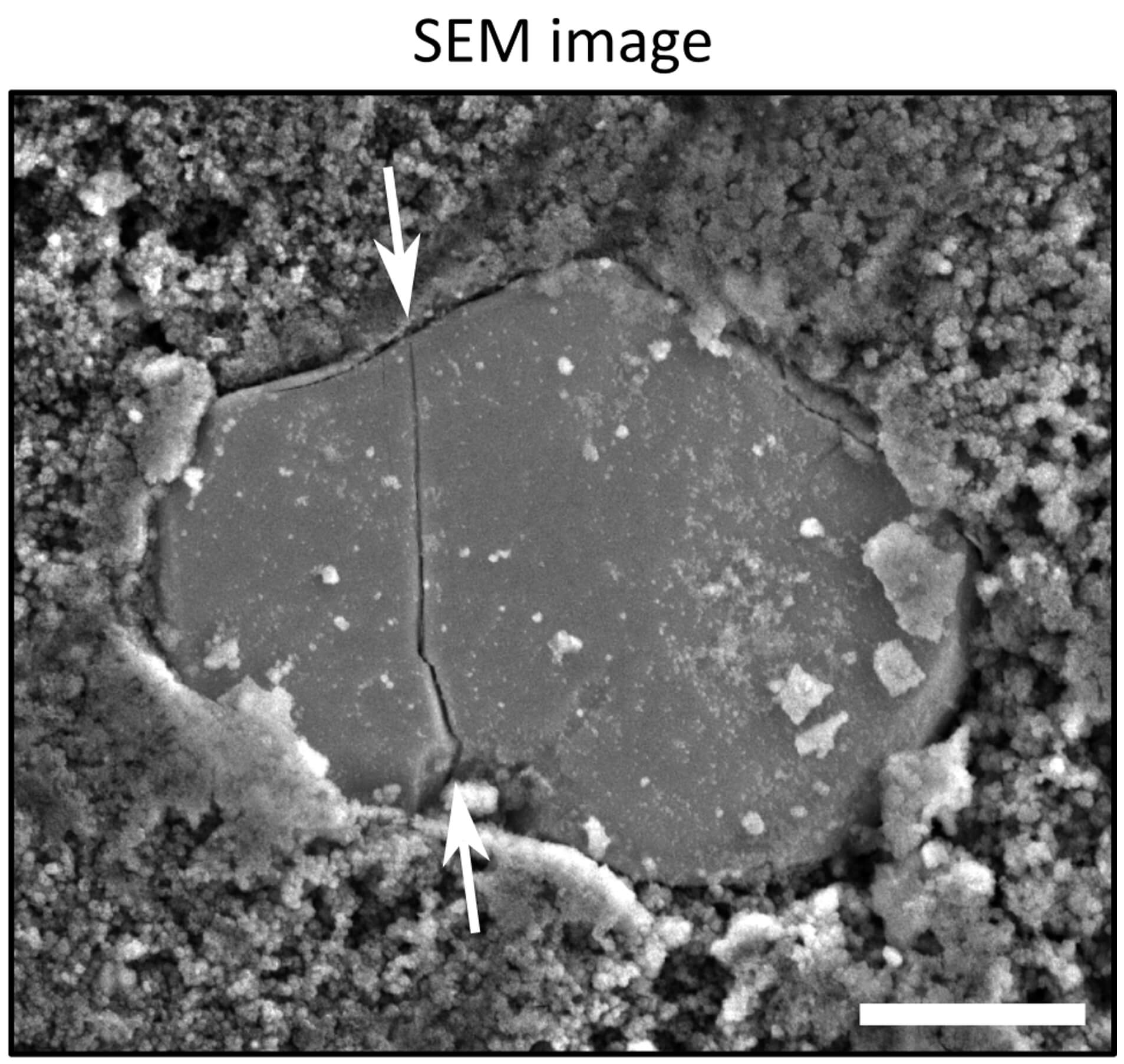
At a faster rate of charge (1C), charge photometry shows that particle fracture occurs during the phase transition. The exact time point and electrochemical conditions when the fracture occurred are captured.
Ensemble electrode-level techniques suggest the NFM phase transition takes place over several hours. Charge photometry shows that the phase transition can take place on a much shorter timescale (minutes vs hours) in some particles, behaviour that would be hidden with ensemble electrode-level approaches.
illumionONE can rapidly identify those cathode materials with properties best suited to your needs.
Charge photometry:
Use charge photometry to understand material properties to enhance performance